Methods for aggregation and differentiation of magnetic stem cells for cartilage recovery purposes
Référence
04222-01
Statut des brevets
French priority patent application n° FR11 57755 filed on September 1st, 2011 and entitled » Procédés d’agrégation et de différenciation de cellules souches magnétisées »





Inventeurs
Claire Wilhelm-Hannetel
Delphine Fayol
Nathalie Luciani
Florence Gazeau
Catherine Levisage
Statut commercial
Exclusive or non-exclusive license
Laboratoire
Laboratoire Matière et Systèmes Complexes (MSC, UMR7057), Paris, France
Description
CONTEXT
Tissue engineering lies at the crossroads of life sciences and mechanical engineering. Although it has the potential to repair or replace defective tissues, current approaches fail to meet clinical demands: therapeutic cells are difficult to retain at the target site, and available biomaterials do not provide an ideal scaffold for structural and functional organisation. One challenge in tissue engineering is thus to produce thick, organized tissues without using an artificial supporting matrix.
Clever biophysical approaches to making artificial constructs that mimic the structural complexity of native tissues typically use (nano)patterning of the substrate, but only provide 2D environments and depend on adhesion to the substrate. Cell seeding in a 3D scaffold can be used as an alternative to an extracellular matrix, and such bioengineered tissues have been successfully implanted in various animal models to restore or improve original tissue functions. However, artificial scaffolds still raise difficult issues of toxicity, degradation, mechanical mismatch and contractility (for cardiac muscle or vascular structures). Other techniques have been developed to engineer scaffold-free tissues composed of cells and the extracellular matrix they secrete. Bioprinting and cell sheet technology both have the potential to control the geometry of a tissue construct. The magnetic aggregate formation developed in the patent is a third option to shape a cell assembly.
It is particularly well suited for stem cells differentiation into chondrocytes (chondrogenesis), to build a functional cartilage tissue. Indeed chondrogenesis requires a preliminary critical step of cell condensation. Using a condensation by centrifugation has demonstrated a limit in the number of compacted cells to form a viable cartilage tissue (over 250000 cells, a central necrosis occur, and chondrogenesis is impacted). Besides, an aggregate shaped from 250000 cells by centrifugation has a size below 1mm, and a round shape.
By contrast, magnetic compaction of mesenchymal stem cells (MSC) yield a very thick cell layer rich in cartilage matrix with no size limit and a totally flexible geometry.
TECHNICAL DESCRIPTION
The approach consists of forming cartilage precursors between 0.5 and 1 mm thick from stem cells aggregated with miniaturized magnets, then merging them successively to obtain a layer of the same thickness (0.5-1 mm) with that can be adapted to fit any defect in patients’ native cartilage.
Figure 1 shows an example of compacted magnetic stem cells, followed by tissue construction under magnetic control.
Labeling of cells with magnetic nanoparticles thus allows controlling size, shape, and timing of compaction.
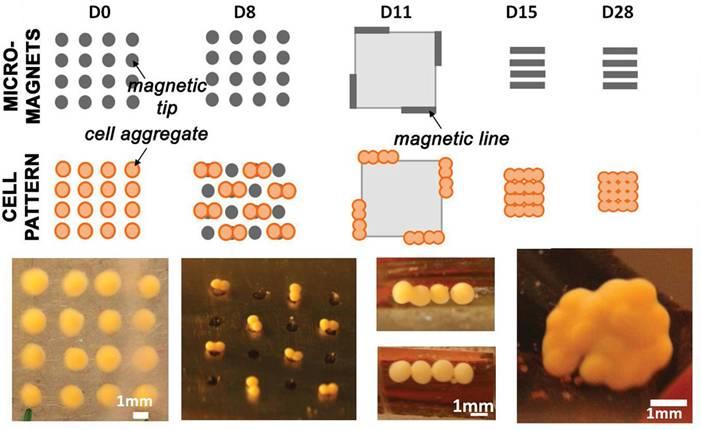
Figure 1: Miniaturized magnets confine stem cells into cartilage building blocks and fuse these blocks into a larger structure rich in collagen
DEVELOPMENT STAGE
Figure 2 illustrates the efficiency of the approach, resulting in a thick cartilage layer rich in proteoglycans.
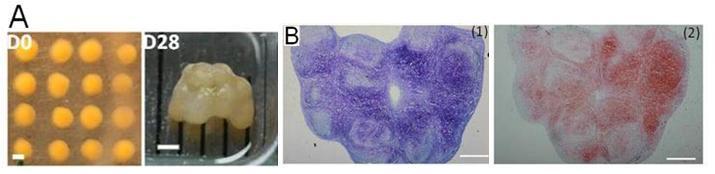
Figure 2: The large structure obtained after fusion of aggregates (A), is rich in proteoglycans (B), essential constituent of cartilage.
Figure 3 confirms by genetic expression the impressive increase in aggregan and collagen II of the magnetically produced tissue.
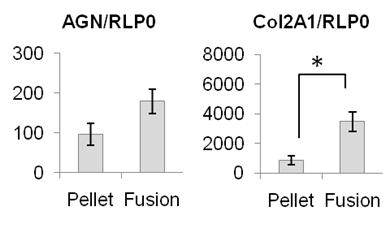
Figure 3: Expression of aggrecan and collagen II – 2 fundamental constituents of cartilage – was increased after magnetic fusion, and results in a large scale cartilage substitute demonstrating excellent levels of collagen II.
Without magnetic control, any structure resulting from the association of such numbers of cells lead to necrotic cellular clusters, with no differentiation within. In this case, a support scaffold would be required for creation of large structure of cartilage. Here, the major advantage is that no scaffold is needed, for this new technique of in vitro cartilage production.
BENEFITS
The magnetic cartilage engineering technology succeeded in providing adjustable tridimensional structures with no size limit. The flexible geometry can be modulated at will in order to exactly fit one patient’s defect.
The use of autologous stem cells will avoid any rejection issue. Importantly, we demonstrated that the iron labeling of stem cells didn’t display any toxicity.
For further information, please contact us (Ref 04222-01)
Besoin de plus d'informations ?
Nous contacterTechnologies Liées
-
29.09.2014
A new additive to enhance power for batteries or enhance energy for supercapacities
Santé / Thérapeutique 04216-01
-
24.02.2014
New set of mast cell lines to stuty allergic and inflammatory mechanisms, as well as oncogenic events
Santé / Thérapeutique, Diagnostic médical, Outils de recherche et criblage 04676-01
-
03.10.2013
Use of cells derived from adipose tissue for antitumoral treatment
Santé / Thérapeutique 00611-01